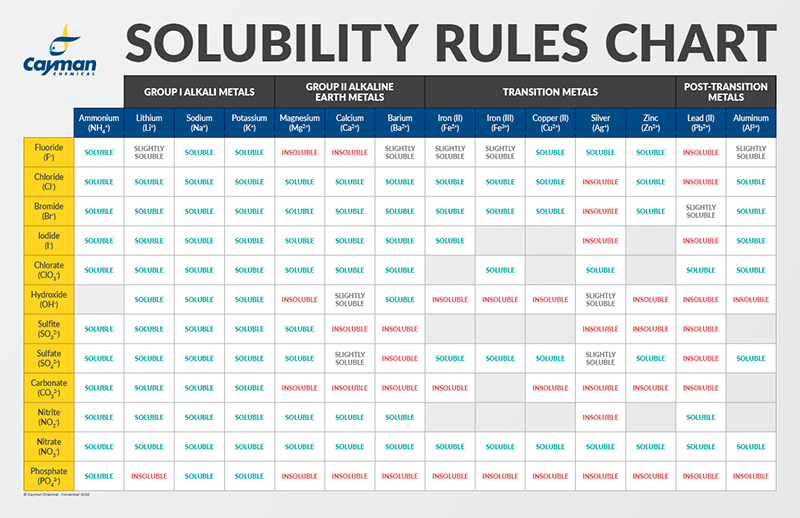
Estimation of the solubility and long-range interaction of the polymer... | Download Scientific Diagram

Draw the structures of ethanol, acetone, toluene, hexane, and water. Classify each solvent as polar, nonpolar, or moderately polar. | Homework.Study.com

Measurement and Correlation of Solubility of Ropivacaine in Four Binary Solvents from 278.15 K to 318.15 K | Journal of Chemical & Engineering Data
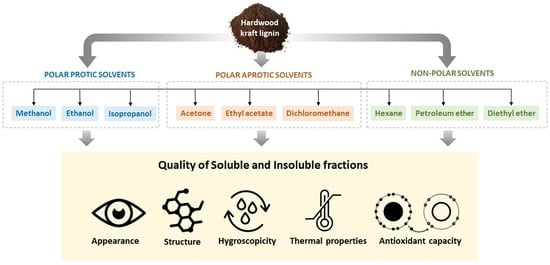
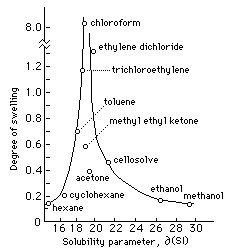
Polymers | Free Full-Text | One-Step Lignin Refining Process: The Influence of the Solvent Nature on the Properties and Quality of Fractions
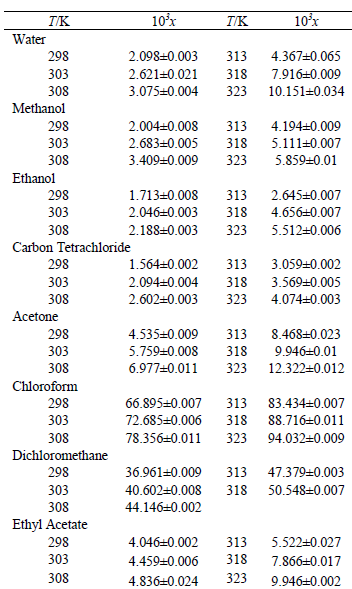
Solubility of caffeine in water, ethyl acetate, ethanol, carbon tetrachloride, methanol, chloroform, dichloromethane, and acetone between 298 and 323 K
Solubilities of some normal saturated and unsaturated long-chain fatty acid methyl esters in acetone, n-hexane, toluene, and 1,2-dichloroethane | Journal of Chemical & Engineering Data
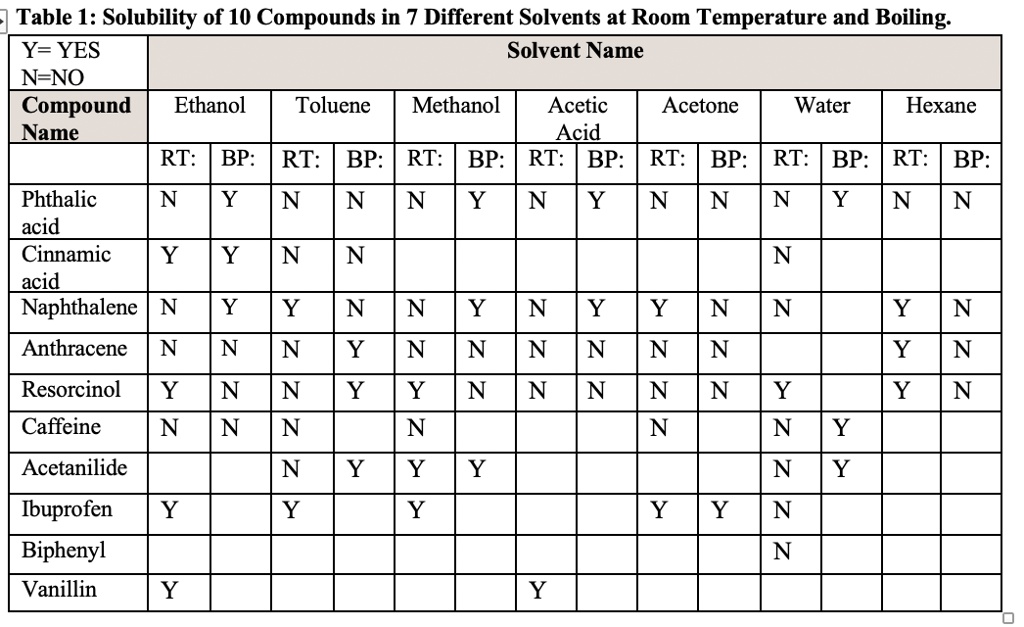
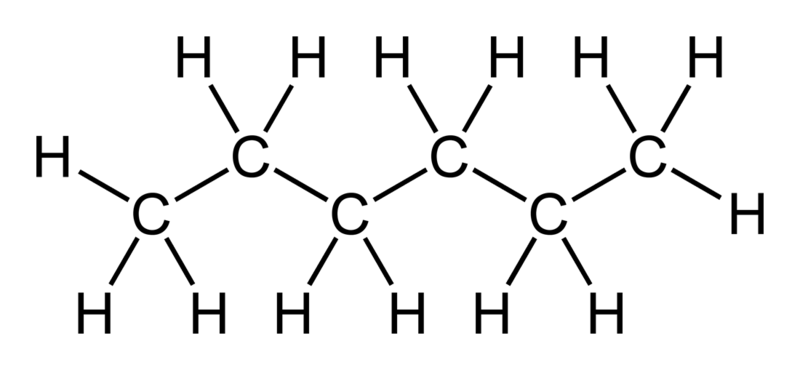
SOLVED: Table I: Solubility of 10 Compounds in 7 Different Solvents at Room Temperature and Boiling Point Compound Solvent Name Ethanol Toluene Methanol Acetic Acid Acetone Water Hexane NNQ Phthalic Acid N N N N Y N N
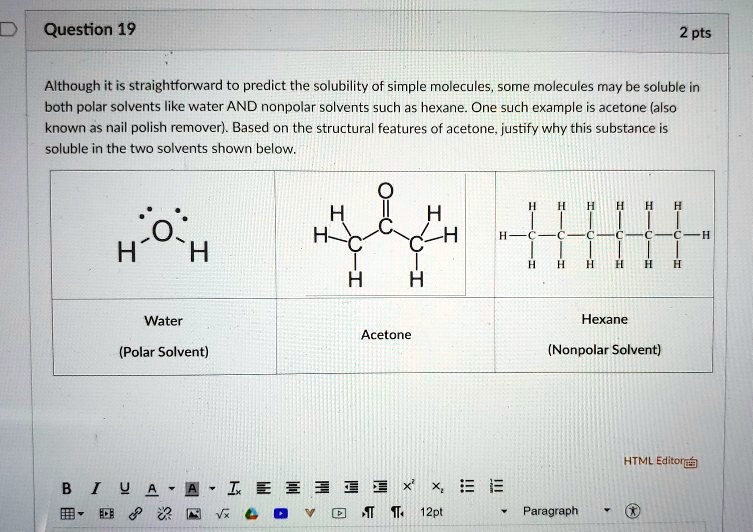
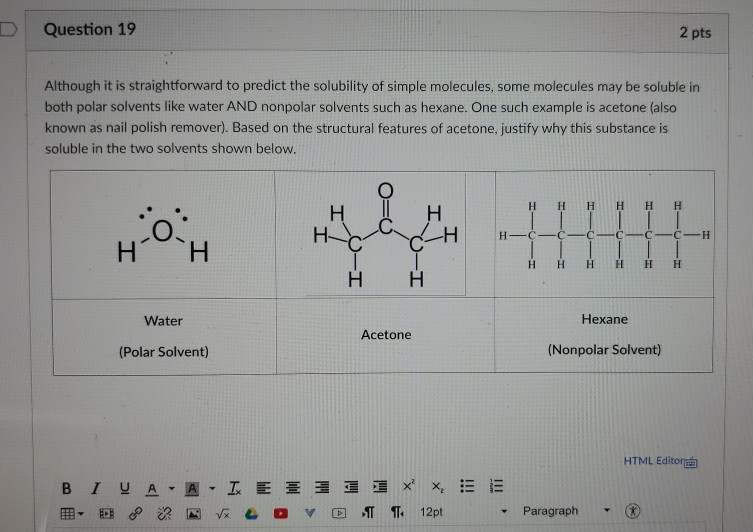
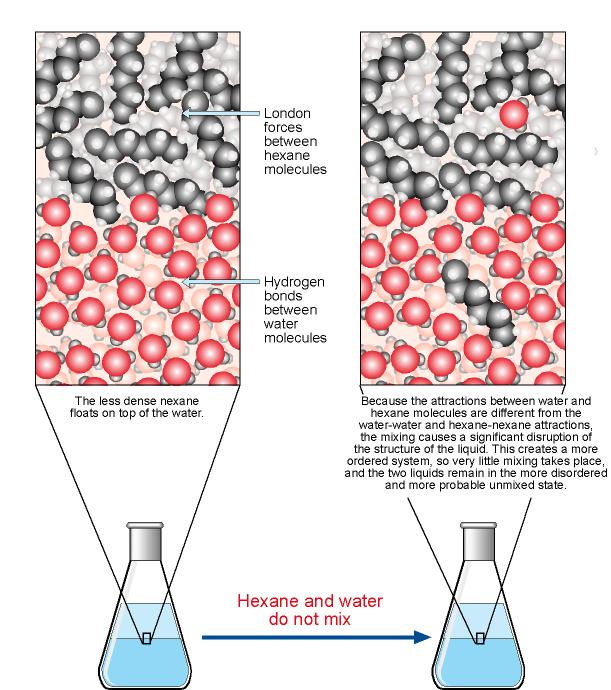
SOLVED: Although it is straightforward to predict the solubility of simple molecules, some molecules may be soluble in both polar solvents like water and nonpolar solvents such as hexane. One such example

SOLVED: Although it is straightforward to predict the solubility of simple molecules, some molecules may be soluble in both polar solvents like water and nonpolar solvents such as hexane. One such example